Organizations in healthcare, finance, pharmaceuticals, and government face a critical challenge: 65% cite data security as a top barrier to AI adoption, yet the competitive pressure to deploy AI capabilities continues to intensify. The MCP Gateway solves this by providing SOC2 Type II-attested infrastructure that transforms local MCP servers into production-ready services with OAuth protection, complete audit trails, and enterprise-grade monitoring—all deployed in minutes, not months.
Key takeaways
- Compliance-First Architecture: MCP's standardized protocol enables granular access controls and comprehensive audit trails required for SOC2, PCI-DSS, and FDA regulations without custom integration development
- Faster Deployment in Controlled Environments: Organizations using standardized protocols reduce implementation time by 40% while maintaining regulatory validation requirements
- Data Sovereignty Without Sacrifice: MCP servers operate within secure networks, ensuring regulated data never leaves approved boundaries, while AI applications receive the necessary context
- Reduced Long-Term Integration Costs: Build validated MCP servers once and reuse them across multiple AI applications, eliminating the need for custom integrations that cost financial services firms millions of dollars annually on compliance technology
- Production-Ready Security: Implement zero-trust architectures where each AI request is validated against current access policies, supporting the 58% improvement in response time reported by organizations with standardized AI integration protocols
1. Clinical decision support with EHR access
Healthcare providers face a fundamental tension: clinicians need AI assistants that access complete patient histories for accurate recommendations, yet healthcare data breaches cost an average of $10.22 million, the highest of any industry. MCP servers provide the answer by enabling secure, controlled access to electronic health records.
How IT works
The MCP Gateway wraps MCP servers with OAuth 2.0 and SAML authentication, ensuring only authorized clinicians have access to patient data through AI assistants. Each query is logged with complete audit trails showing who accessed what information, when, and for what purpose.
Implementation example
A multi-system healthcare network deploys MCP servers within its existing security perimeter, integrating with its enterprise authentication system. When a clinician asks Claude Desktop about a patient's lab results, the request:
- Authenticates the clinician's credentials against Active Directory
- Verifies the clinician has an active treatment relationship with the patient
- Queries only the specific EHR data needed (minimum necessary standard)
- Logs the complete interaction with timestamp, user ID, and data accessed
- Returns results directly to the clinician's AI assistant
Regulatory benefits
- Security Rule Satisfaction: Implements required access controls, audit trails, and encryption in transit
- BAA-Ready Architecture: BAA readiness requires contractual BAAs plus technical safeguards (access control, audit logging, encryption)
Business impact
The majority of Healthcare IT leaders report that interoperability challenges as barriers to clinical AI deployment. MCP's standardized approach eliminates these barriers while maintaining the security controls that protect against the $10.22 million average breach cost.
Getting Started: Deploy the Elasticsearch MCP Server for AI-powered knowledge base search across medical documentation, or integrate custom MCP servers with your EHR systems using MCP Gateway's hosted deployment options.
2. Fda-compliant drug development documentation
Pharmaceutical companies operate under some of the strictest validation requirements in any industry, with a significant amount of their revenue dedicated to compliance and validation activities. AI assistance with regulatory submissions could significantly reduce this burden—but only if the systems handling electronic records meet FDA 21 CFR Part 11 requirements for audit trails, electronic signatures, and system validation.
The regulatory challenge
Traditional API integrations between AI applications and document management systems require individual validation for each connection, creating months of work for quality assurance teams. Any change to the integration invalidates the validation, forcing a complete re-validation cycle.
MCPs validation advantage
Deploy MCP servers that connect to validated document management systems through a single, validated interface. The MCP server itself undergoes validation once, then multiple AI applications can access it without requiring separate validation for each application.
Use case implementation
A pharmaceutical company implementing AI assistance for regulatory submissions:
- Phase 1: Deploy an MCP server providing controlled access to their validated document management system (eDMS)
- Phase 2: Validate the MCP server's access controls, audit logging, and data integrity mechanisms
- Phase 3: Connect multiple AI applications (writing assistants, search tools, data extraction) to the validated MCP server
- Result: Each new AI application requires configuration rather than full validation, reducing deployment time from 6-12 months to weeks
Audit trail requirements met
The MCP Gateway provides complete logs showing:
- User identification and authentication
- Date and time stamps for all document access
- Reason for record changes or access
- Previous values for any modified data
- System-generated audit trail that cannot be altered
Compliance documentation
Organizations receive architecture diagrams, data flow documentation, security configurations, and change control records needed for FDA inspections—all maintained automatically by MCP Gateway's audit and observability features.
Cost Impact: By reducing validation cycles and enabling reusable infrastructure, pharmaceutical companies can redirect portions of their compliance IT budget toward innovation rather than repetitive validation work.
3. PCI-DSS compliant fraud detection for financial services
Financial institutions face a perfect storm, with a lot of reports indicating increased regulatory scrutiny of AI and machine learning systems, while fraud losses demand increasingly sophisticated detection capabilities. Traditional approaches that provide AI systems with broad access to transaction data create unacceptable compliance risks under PCI-DSS requirements.
The PCI-DSS challenge
Payment Card Industry Data Security Standard mandates strict controls over cardholder data access, including:
- Network segmentation isolating cardholder data
- Granular access controls limiting data access to business needs
- Comprehensive logging of all access to cardholder data
- Encryption of data in transit and at rest
MCP solution architecture
The Snowflake MCP Server enables banks to provide AI fraud detection systems with transaction pattern analysis capabilities while maintaining complete PCI-DSS compliance:
Implementation Details:
- Data Segmentation: MCP servers operate within the cardholder data environment (CDE), ensuring AI applications never receive raw cardholder data
- Query Restrictions: Configure MCP servers to execute only pre-approved analytical queries that return aggregated patterns, not individual card numbers
- Access Logging: Every AI query is logged with user identity, timestamp, query executed, and data returned
- Encryption: All communications between AI applications and MCP servers use TLS 1.3 encryption
Practical fraud detection workflow
When an AI system analyzes potential fraud:
- The system authenticates through the MCP Gateway's OAuth integration
- It requests transaction patterns for a specific merchant category or geographic region
- The MCP server executes the query against Snowflake, returning only aggregated statistics
- The AI identifies anomalies without ever accessing individual cardholder data
- Complete audit trail documents the analysis for compliance review
Regulatory Benefits:
- Req 3.5.1: PAN rendered unreadable at rest.
- Req 7: Restrict access by business need-to-know.
- Req 10: Log and monitor all access.
- Req 4: Strong crypto in transit.
Business impact
Organizations implementing MCP-based fraud detection maintain a good percentage of reduction in security incidents associated with standardized protocols while meeting the intensive logging requirements that financial regulators demand.
4. Glba-compliant customer service with data protection
Banks providing AI-powered customer service face strict requirements under the Gramm-Leach-Bliley Act privacy provisions. Customer service representatives need account information to assist customers, but AI systems accessing this data create new privacy and security risks that most CIOs identify as critical concerns.
GLBA privacy requirements:
- Notice: Customers must be informed about information collection and sharing
- Opt-Out Rights: Customers must have the ability to limit information sharing
- Safeguards: Financial institutions must protect customer information security
- Pretexting Protection: Prevent fraudulent information access
MCP solution for customer service
Deploy MCP servers that provide AI customer service assistants with controlled access to customer relationship management systems while implementing data masking, purpose limitation, and consent verification.
Implementation architecture
Data Access Controls:
- MCP servers implement field-level security, returning only information necessary for the specific customer inquiry
- Social Security numbers, account numbers, and other sensitive data are masked unless specifically required
- Access requires both customer service representative authentication and customer consent verification
Consent Management Integration:
- Before providing customer data to AI assistants, MCP servers verify that customers have consented to AI-assisted service
- Customers who opt out of AI assistance receive traditional service, with MCP servers returning no data to AI applications
- All consent decisions are logged for regulatory compliance demonstration
Purpose Limitation Enforcement:
- MCP Gateway's tool governance features restrict which CRM operations AI assistants can perform
- Read operations are permitted for customer service; write operations require human approval
- Marketing inquiries are blocked—data can only be accessed for authorized customer service purposes
Practical customer service workflow
When a customer service representative using an AI assistant helps a customer:
- Representative authenticates through enterprise SSO
- Customer identity is verified through standard bank procedures
- AI assistant requests customer account information through the MCP server
- MCP server verifies consent status and purpose of access
- Only necessary account details (current balance, recent transactions) are returned with sensitive data masked
- Complete interaction is logged for privacy compliance audits
Privacy compliance documentation
Organizations receive audit trails demonstrating:
- Customer consent was verified before data access
- Only authorized representatives accessed customer information
- Data access was limited to legitimate customer service purposes
- Opt-out preferences were honored systematically
Business impact
Financial institutions maintain the customer service efficiency gains from AI assistance while satisfying the privacy controls that a lot of financial institutions report are now under increased regulatory scrutiny.
5. ISO 13485 quality management for medical device manufacturers
Medical device manufacturers face stringent quality management system requirements under FDA QSR and ISO 13485 standards. AI assistance with quality investigations, corrective and preventive actions (CAPA), and design history files could significantly improve efficiency—but only if the systems maintain the document control and record retention requirements that regulators demand.
Quality system record requirements:
- Document Control: Version control, approval workflows, and change history for all quality documents
- Record Retention: Complete history of quality records for device lifetime plus additional years
- Audit Trails: Who created, modified, or accessed quality records and when
- Electronic Signatures: Validated electronic signatures for quality document approval
MCP implementation for quality management
Deploy MCP servers providing controlled access to quality management systems (QMS) while maintaining 21 CFR Part 820 and ISO 13485 compliance:
Architecture for validated systems
Document Access Controls:
- MCP servers integrate with validated QMS platforms (MasterControl, Veeva Vault, TrackWise)
- Role-based access ensures only authorized quality personnel have access to specific document types
- Read-only access for AI assistants unless write operations receive human approval
- All document access is logged with user identity, timestamp, and business purpose
Electronic Record Integrity:
- MCP servers return documents with complete metadata (version, approval status, effective date)
- AI-generated content is marked as draft pending human review and approval
- No modifications to controlled documents occur without validated electronic signature workflows
Use case: CAPA investigation support
A medical device manufacturer implements AI assistance for CAPA investigations:
Traditional CAPA Process:
- Quality engineers manually search for similar previous CAPAs
- Root cause analysis requires reviewing multiple quality record types
- Investigation takes 2-4 weeks on average
- No systematic way to identify patterns across similar investigations
MCP-Enhanced CAPA Process:
- Quality engineers describe the nonconformance to AI assistants
- Elasticsearch MCP Server searches historical CAPA records, complaint data, and nonconformance reports
- AI identifies similar previous investigations and their root causes
- Quality engineer reviews suggestions and completes the investigation in 3-5 days
- All AI queries and data access are logged for an audit trail
Validation documentation
Organizations implementing MCP for quality management receive validation packages, including:
- System Architecture: Data flow diagrams showing how MCP servers integrate with validated QMS
- Access Control Matrix: Role-based permissions and tool access by quality function
- Audit Trail Reports: Evidence that all quality record access is logged completely
- Change Control Procedures: Documented processes for MCP configuration changes
Regulatory benefits:
- 21 CFR Part 11 Compliance: Audit trails, electronic signatures, and access controls meet FDA requirements
- ISO 13485:2016 Clause 4.2.4: Document control and record requirements are maintained
- FDA Inspection Readiness: Complete audit trails demonstrate quality system oversight
Efficiency gains
By reducing CAPA investigation time from weeks to days while maintaining regulatory compliance, manufacturers can allocate quality resources to prevention activities rather than reactive investigations—improving overall product quality and patient safety.
6. Supply chain traceability for regulated manufacturing
Companies in regulated supply chains face unique challenges: they must trace materials from supplier to finished product while maintaining data integrity requirements, but supply chain data is distributed across multiple systems and external partners. FDA supplier controls and EU MDR traceability requirements demand comprehensive documentation that traditional systems struggle to provide.
Regulatory traceability requirements:
- Material Traceability: Track raw materials from the supplier through finished products
- Certificate of Analysis: Maintain testing documentation for all incoming materials
- Supplier Qualification: Document supplier approval and ongoing monitoring
- Chain of Custody: Prove continuous control from receipt through distribution
MCP implementation for supply chain intelligence
Deploy MCP servers that provide AI assistants with controlled access to supply chain systems (ERP, quality management, supplier portals) while maintaining data integrity and audit requirements:
Architecture components
Multi-System Integration:
- MCP servers connect to ERP systems (SAP, Oracle), QMS platforms, and supplier qualification databases
- Database MCP servers enable natural language queries across distributed supply chain data
- Unified audit trail tracks which systems AI assistants accessed for each query
Data Integrity Controls:
- MCP servers provide read-only access to validated records
- Any data modifications require human approval through established change control processes
- Audit trails demonstrate no unauthorized changes to supply chain records
Use case: Rapid response to material quality issues
A pharmaceutical manufacturer discovers a quality issue with an active pharmaceutical ingredient (API):
Traditional Investigation Process:
- Quality engineers manually search the ERP system for all lots using the suspect API
- Search supplier qualification records to identify potential root causes
- Review certificates of analysis for incoming material testing
- Contact manufacturing sites to identify affected finished product lots
- Investigation requires 2-3 days with multiple quality engineers
MCP-Enhanced Investigation:
- The quality engineer describes the API lot number to the AI assistant
- Snowflake MCP Server queries integrated the supply chain data warehouse
- AI identifies all finished product lots containing the suspect API within minutes
- Supplier qualification history and recent audit findings are retrieved automatically
- Quality engineer reviews findings and initiates containment actions the same day
- Complete audit trail documents the investigation for regulatory submissions
Regulatory Compliance Benefits:
- FDA 21 CFR Part 820.50: Purchasing controls are maintained with complete traceability
- EU MDR Article 10.8: Traceability requirements are satisfied through comprehensive supply chain documentation
- ISO 13485 Clause 7.4: Supplier management documentation is readily accessible for audits
Efficiency impact
By reducing investigation time from days to hours, manufacturers can implement containment actions faster, reducing the scope of quality issues and protecting patient safety while maintaining the documentation requirements that regulators demand during inspections.
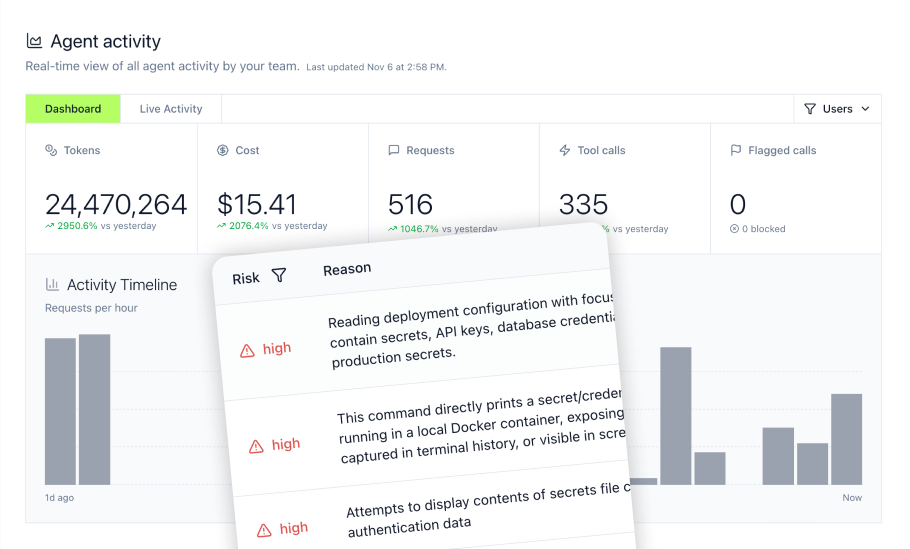
7. Coding agent security in regulated development environments
A staggering 67% of companies in regulated industries cite lack of proper AI governance frameworks as a major challenge—and nowhere is this more acute than in development environments where coding agents like Cursor and Claude Code operate with extensive system access.
The coding agent risk
Development teams increasingly use AI coding assistants, but these tools can:
- Access sensitive configuration files containing credentials and API keys
- Execute bash commands with developer permissions
- Read source code, including proprietary algorithms
- Modify production deployment configurations
- Access customer data in development databases
Why this matters for regulated industries
Organizations under SOC2 or PCI-DSS requirements must demonstrate that sensitive data and credentials are protected from unauthorized access. Without monitoring, organizations cannot prove that AI coding agents haven't exposed credentials or accessed regulated data inappropriately.
LLM proxy solution
The LLM Proxy sits between coding agents and language models, monitoring every MCP tool invocation, bash command, and file access in real-time:
Monitoring Capabilities:
- Tool Call Tracking: See every MCP tool invocation from Cursor, Claude Code, Windsurf, and other coding agents
- Bash Command History: Track all shell commands executed by AI agents, identifying dangerous operations
- File Access Logging: Monitor which files agents read or modify, protecting sensitive configuration
- MCP Inventory: Complete visibility into installed MCPs and their permissions across development teams
Security Guardrails:
- Dangerous Command Blocking: Prevent execution of commands that could expose credentials or damage systems
- Sensitive File Protection: Block access to .env files, SSH keys, cloud credentials, and other sensitive configuration files
- Real-Time Alerts: Notify security teams when agents attempt risky operations
- Policy Enforcement: Define and enforce organizational policies for agent behavior
Use case: Protecting development credentials
A financial services company implements LLM Proxy to protect development infrastructure:
Before LLM Proxy:
- Developers use coding agents with no monitoring or controls
- No visibility into what files agents access or commands they execute
- The compliance team cannot demonstrate credential protection during the SOC2 audit
- Risk that agents might expose AWS keys, database passwords, or API tokens
After LLM Proxy Implementation:
- All coding agent activity is monitored and logged through LLM Proxy
- Attempts to access .env files or credential stores are blocked automatically
- Security team receives alerts when agents attempt dangerous bash commands
- Complete audit trail demonstrates credential protection for compliance review
- Developers maintain productivity with coding agents while security is enforced
Compliance Benefits:
- SOC2 CC6.1: Logical access controls are demonstrated through agent activity monitoring
- PCI-DSS Requirement 7: Access to sensitive data is restricted and monitored
- HIPAA Security Rule: Technical safeguards for electronic PHI access are maintained
Implementation Steps:
- Deploy LLM Proxy using client setup guides
- Configure policies blocking access to sensitive files and dangerous commands
- Integrate with security monitoring platforms for alert notification
- Train development teams on policy rationale and approved workflows
- Review audit logs during regular security reviews
Developer experience
The lightweight service sits between coding agents and LLM providers, forwarding requests transparently. Developers experience no latency impact while organizations gain essential visibility and control over agent behavior that compliance frameworks demand.
Making your choice: Essential considerations
Regulatory requirements first
Before implementing any MCP infrastructure, map your specific regulatory obligations to technical controls. Healthcare organizations need BAA-ready architecture; financial services require SOC2 and PCI-DSS compliance; pharmaceutical companies need FDA validation documentation. The MCP Gateway's SOC2 Type II attestation and comprehensive audit trails provide the foundation, but organizations must configure policies matching their specific requirements.
Data sovereignty vs. Cloud services
The majority of enterprises in regulated sectors preferring on-premises or hybrid AI deployments reflect real compliance constraints. MCP architecture supports both cloud-based and self-hosted deployment, enabling organizations to maintain data within controlled environments while accessing AI capabilities. Government agencies handling classified information, healthcare organizations managing PHI, and financial institutions with data residency requirements can deploy MCP infrastructure entirely within their security perimeters.
Integration complexity reality
Organizations implementing MCP report a significant percentage of reduction in integration development time after initial setup—but that initial setup requires investment. Plan for 2-4 weeks to deploy pilot MCP servers, 4-8 weeks for validation and testing, and 3-6 months for enterprise rollout. Organizations should allocate resources for collaboration between AI/ML teams, security teams, compliance officers, and IT infrastructure teams.
Audit trail completeness
Compliance frameworks universally require comprehensive logging, but organizations using standardized protocols experience fewer security incidents—in part because systematic audit trails enable faster incident detection and response. The MCP Gateway's audit capabilities provide the logs that SOC2 and PCI-DSS auditors demand, but organizations must establish procedures for regular log review and incident response.
Cost-Benefit analysis
Financial services firms spend millions of dollars annually on regulatory compliance technology, while pharmaceutical companies allocate 5-9% of their annual revenue to compliance and validation. MCP infrastructure requires upfront investment but reduces long-term costs through reusable architecture—validated MCP servers support multiple AI applications without separate validation for each integration.
Vendor ecosystem maturity
MCP launched in late 2024, meaning ecosystem maturity, vendor support, and validated implementation patterns are still developing. Organizations should expect to build some capabilities in-house during initial implementation. However, MCP is supported by Anthropic, OpenAI, Google, and Microsoft, suggesting strong ecosystem development is likely.
Frequently asked questions
Q: Can MCP gateway support multi-region deployments with data residency controls?
A: Yes. You can pin deployments to specific regions so regulated data never leaves its jurisdiction, while still serving global apps through region-aware routing and policies.
Q: What MCP examples exist for financial services compliance and reporting?
A: Common patterns include a Snowflake MCP Server for SOX-friendly NL queries with full audit trails, PCI-DSS fraud analysis without exposing PAN data, GLBA-aware customer support with masking/consent checks, and automated regulatory reporting with data lineage.
Q: How does LLM proxy protect sensitive files and credentials in coding workflows?
A: It inspects every MCP/tool call and shell command, blocks reads of secrets (e.g., .env, SSH keys, cloud creds), and stops privileged ops via policy. Alerts and full activity logs support SOC 2 and PCI-DSS reviews.
Q: What audit trail capabilities does MCP gateway provide for SOC 2 audits?
A: It records who accessed what and how (identity, auth method, time, tools, params, results), with tamper-evident logs and policy-aligned retention. Prebuilt reports and SIEM exports streamline auditor review.