Pharmaceutical brands face a unique challenge: some report data integration as their top barrier to AI adoption, yet 92% prioritize AI investments for digital transformation in most sectors. Model Context Protocol (MCP) addresses this gap by providing a standardized infrastructure that connects AI assistants to clinical databases, regulatory systems, and commercial platforms while maintaining the compliance and security that pharmaceutical operations demand. The MCP Gateway enables pharmaceutical teams to deploy AI tool access efficiently, with enterprise-grade governance built in from day one.
Key takeaways
- Model Context Protocol (MCP) enables pharmaceutical brands to connect AI assistants to fragmented data systems without costly custom integrations, addressing the challenge of managing dozens of disconnected platforms across operations
- AI-enabled process improvements in life sciences have delivered good efficiency gains in information-intensive functions, while MCP infrastructure supports SOC2 Type II audited controls and safeguards when handling protected health information
- High-value use cases span clinical data analysis, regulatory compliance, medical information services, pharmacovigilance monitoring, and commercial operations—each deployable with standardized infrastructure
- MCP Gateway provides streamlined deployment workflows, OAuth protection, and complete audit trails that transform local MCP servers into production-ready services for pharmaceutical teams
What is MCP and why pharmaceutical marketing teams need IT
Model Context Protocol is an open-source standard proposed by Anthropic that enables AI assistants like Claude, ChatGPT, and Microsoft Copilot to securely connect to external data sources and tools through standardized server implementations. For pharmaceutical brands managing largely unstructured, siloed data across departments, MCP provides the universal integration layer that eliminates costly custom API development.
How MCP connects AI assistants to pharmaceutical data
Traditional pharmaceutical system integration can take many months and a significant investment per major connection. MCP transforms this by providing standardized servers that expose data through a common protocol, allowing multiple AI clients to access the same sources without redundant integration work.
Key architectural benefits for pharmaceutical operations:
- Single integration, multiple AI clients: Connect once to Snowflake, Elasticsearch, or Salesforce; access from Claude Desktop, ChatGPT, Cursor, and custom agents
- STDIO server hosting: Deploy local MCP servers to production with automatic OAuth wrapping and enterprise monitoring
- Audit-ready by design: Complete logging of every AI interaction, data access, and tool invocation supporting 21 CFR Part 11 compliance when implemented with appropriate validation
- Granular access control: Configure tool permissions by role, enabling read-only operations while blocking write access to production systems
The compliance challenge in pharmaceutical AI adoption
Pharmaceutical AI implementations must satisfy regulatory requirements that extend beyond typical enterprise security. Data integrity principles such as ALCOA+ (Attributable, Legible, Contemporaneous, Original, Accurate, plus Complete, Consistent, Enduring, Available) are recognized in GxP guidance and align with Part 11 expectations for electronic records. MCP Gateway addresses these requirements through:
- SOC2 Type II audit completion when handling protected health information
- Complete audit logs capturing user identity, timestamp, data accessed, and AI outputs
- OAuth and SAML integration for enterprise authentication across all MCP connections
- Data residency controls ensure sensitive pharmaceutical data remains in approved geographic regions
Medical affairs teams currently spend most of their time searching for information across multiple systems. MCP-connected AI assistants eliminate this inefficiency while maintaining the governance that pharmaceutical operations require.
1. Ai-powered clinical data analysis and regulatory compliance
Pharmaceutical development teams invest an estimated $2.6 billion over 10-15 years developing a single drug, with clinical trial data and regulatory documentation scattered across electronic data capture (EDC) platforms, clinical trial management systems (CTMS), document repositories, and data warehouses. Snowflake MCP Server enables natural language queries of clinical data repositories, while Elasticsearch MCP Server transforms regulatory knowledge management.
Natural language clinical data queries
The Snowflake MCP Server provides pharmaceutical-grade data access through specialized tools, including Cortex Analyst (converts natural language questions into SQL queries), Run Snowflake Query (executes SQL directly with audit trails), and Query Semantic View (accesses pre-configured views with role-based filtering).
Example queries pharmaceutical teams run daily:
- "Show me protocol deviation rates by site for the last quarter."
- "Compare adverse event frequencies between treatment arms"
- "Identify patients meeting eligibility criteria for upcoming trials"
- "Calculate screen failure rates by investigator site"
These queries previously required biostatistics teams to write custom SQL, compile reports, and distribute findings—a cycle taking days or weeks. With MCP-connected AI, clinical research associates get instant answers while maintaining complete traceability of data sources.
Regulatory knowledge base and document access
Large pharmaceutical companies manage hundreds of regulatory submissions annually across different markets, each requiring precise references to SOPs, quality documents, previous approvals, and FDA guidance. The Elasticsearch MCP Server enables semantic search across documentation repositories through tools for query execution, index discovery, and field mapping retrieval.
Knowledge base applications:
- Regulatory affairs: Instant access to historical submission documents, approval letters, and agency correspondence
- Quality assurance: Search across validation protocols, change control records, and deviation reports for CAPA investigations
- Compliance training: Query policy documents, SOPs, and training materials without manual document hunting
Implementation requirements:
- Document indexing in Elasticsearch with appropriate field mappings for metadata (document type, approval date, region, therapeutic area)
- Role-based access control ensures sensitive regulatory strategy documents have restricted visibility
- Integration with existing document management systems (Veeva Vault, SharePoint) through MCP Gateway's hosted connectors
Deployment timeline: 3-6 months for core clinical analytics and regulatory knowledge base with role-based access and validation protocols for GxP compliance
Who benefits most: Clinical operations teams, biostatisticians, clinical research associates, regulatory affairs specialists, quality assurance teams, and compliance officers
2. Medical information services and pharmacovigilance
Pharmaceutical medical information departments handle thousands of healthcare provider inquiries annually, while pharmacovigilance teams monitor safety signals across multiple data sources. The FDA's FAERS database receives over 1.5 million adverse event reports annually, with regulatory requirements mandating 15-day reporting for serious, unexpected adverse events (21 CFR 314.80, 600.80). Gmail MCP Server, combined with Elasticsearch knowledge bases, enables medical affairs teams to transform response workflows and safety monitoring.
Ai-assisted medical information response drafting
The Gmail MCP Server provides five tools for controlled email workflow automation: Search Email, Get Email, Draft Email, Draft Reply, and Send Draft. Traditional medical information responses require 48-72 hours as specialists search multiple databases, compile relevant studies, ensure regulatory compliance, and draft responses. MCP-connected AI assistants retrieve relevant clinical data, safety information, and approved language in seconds, allowing medical reviewers to focus on validation rather than information gathering.
Security controls for protected health information:
- OAuth-based Gmail access: Individual user authentication with revocable tokens, not shared credentials
- Sensitive file protection: Block AI access to attachments containing patient identifiers or proprietary data
- Complete email logging: Capture which medical information specialist accessed each inquiry, what data sources were queried, and the final approved response
Adverse event detection and safety signal monitoring
When healthcare providers report suspected adverse events via email, pharmaceutical companies must meet strict reporting timelines. The combined Gmail + Elasticsearch MCP integration enables automatic triage (search email for adverse event indicators and route to pharmacovigilance teams), historical case lookup (query safety databases for similar event patterns), response template retrieval (access approved language for acknowledgment), and audit trail generation.
AI assistance for pharmacovigilance tasks:
- MedDRA coding suggestions: Analyze case narratives and suggest appropriate Medical Dictionary for Regulatory Activities (MedDRA) terms
- Causality assessment support: Retrieve similar historical cases and relevant clinical literature
- ICSR generation: Pre-populate Individual Case Safety Report fields from structured data sources
- Safety signal detection: Semantic search across published case reports, clinical studies, and regulatory databases
MCP Gateway provides safeguards that pharmacovigilance data requires, including protected health information controls, complete audit trails for regulatory inspection, role-based access (drug safety physicians access full case details; analysts see de-identified aggregate data), and data residency controls.
Deployment timeline: 3-5 months, including pharmacovigilance system integration
Who benefits most: Medical information specialists, medical science liaisons, drug safety physicians, pharmacovigilance specialists, and regulatory reporting coordinators
3. Commercial operations and market intelligence
Pharmaceutical brand managers require real-time access to prescription data, market share trends, payer coverage decisions, and competitive intelligence to make strategic decisions. With brands managing data across a dozen or more different CRM databases, fragmented information delays critical decisions.
Ai-driven territory analytics and sales enablement
Commercial operations teams use Snowflake MCP Server to provide field representatives with real-time insights through natural language queries:
- Territory performance: "Which prescribers in my territory increased Product X prescriptions by more than 20% this quarter?"
- Targeting optimization: "Identify high-potential prescribers based on patient panel demographics and current prescribing patterns for similar medications"
- Call planning intelligence: "Show me the last five interactions with Dr. Smith, including medical information requests and sample deliveries."
- Competitive intelligence: "Which prescribers switched patients from our product to Competitor Z in the last 60 days?"
Sales representatives equipped with integrated AI assistants spend less time on administrative tasks and more time on productive healthcare provider engagement. Improved call quality correlates with increased provider satisfaction and prescription lift compared to traditional detailing approaches.
Brand performance analytics and market access
Critical data sources for commercial effectiveness:
- Prescription data: IQVIA, Symphony Health, or proprietary data warehouses tracking prescribing patterns
- Payer coverage: Formulary status, prior authorization requirements, and reimbursement changes
- Patient support programs: Copay card availability, patient assistance eligibility, and enrollment status
- Medical information: Approved responses to common prescriber questions about efficacy, safety, and dosing
Commercial analytics teams create Snowflake semantic views defining key performance indicators (market share metrics, representative activity, incentive compensation, customer segmentation). Product management teams using AI-driven analytics generate instant insights through natural language queries rather than waiting for scheduled reports from business intelligence teams.
Deployment timeline: 2-4 months for commercial operations pilot with core CRM integration
Who benefits most: Sales representatives, district managers, brand managers, marketing strategists, commercial analytics teams, and market access professionals
4. Medical affairs and clinical development
Medical science liaisons (MSLs) and clinical development teams operate at the intersection of clinical evidence, healthcare provider education, and trial execution. MSLs currently spend significant time searching for information—time better spent on strategic engagement. Clinical trial patient recruitment failures cost the pharmaceutical industry $600k to 8 million per day in delayed approvals.
Ai-powered literature review and scientific communications
Elasticsearch MCP Server enables continuous monitoring of PubMed and clinical trial registries (track new publications related to therapeutic areas and competitor products), medical conference proceedings (monitor abstracts from major congresses like ASCO, ASH, ADA, ACC), regulatory guidance updates (alert teams to FDA/EMA policy changes), and investigator-sponsored trials (identify real-world evidence relevant to brand strategy).
MCP-enabled MSL workflows:
- Pre-call planning: Query Snowflake for healthcare provider prescribing patterns, therapeutic area focus, and previous interaction history
- Scientific inquiry response: Access approved clinical study summaries, publication reprints, and medical information letters from Elasticsearch repositories
- Congress preparation: Retrieve abstract presentations, investigator profiles, and key data points for upcoming medical conferences
- Evidence synthesis: Compile clinical evidence across multiple studies for healthcare provider education
Clinical trial site selection and operations
Snowflake MCP Server enables clinical development teams to query trial feasibility databases with natural language for site selection ("Which investigator sites have enrolled more than 50 cardiovascular patients in the last 18 months with retention rates above 90%?"), enrollment projections ("Estimate patient population meeting eligibility criteria based on electronic health record data in target geographies"), and trial monitoring ("What's the current enrollment rate across all sites, and which sites are below target?").
Data sources for clinical development MCP integration:
- Clinical trial management systems (CTMS): Medidata Rave, Oracle Siebel CTMS, Veeva Vault CTMS
- Electronic data capture (EDC): Medidata Rave EDC, Oracle Clinical, REDCap
- Patient recruitment databases: Trial enrollment platforms, patient registries, investigator site networks
- Real-world data: Electronic health records, claims databases for feasibility and site identification
Clinical operations teams can reduce trial timelines through improved patient identification, automated protocol deviation detection, and predictive analytics for enrollment challenges.
Deployment timeline: 3-6 months for medical affairs knowledge base with literature monitoring and clinical trial analytics, including validation for GxP compliance
Who benefits most: Medical science liaisons, medical communications specialists, publication planning teams, clinical operations managers, clinical research associates, and patient recruitment specialists
Monitoring and governance: Essential controls for pharmaceutical AI
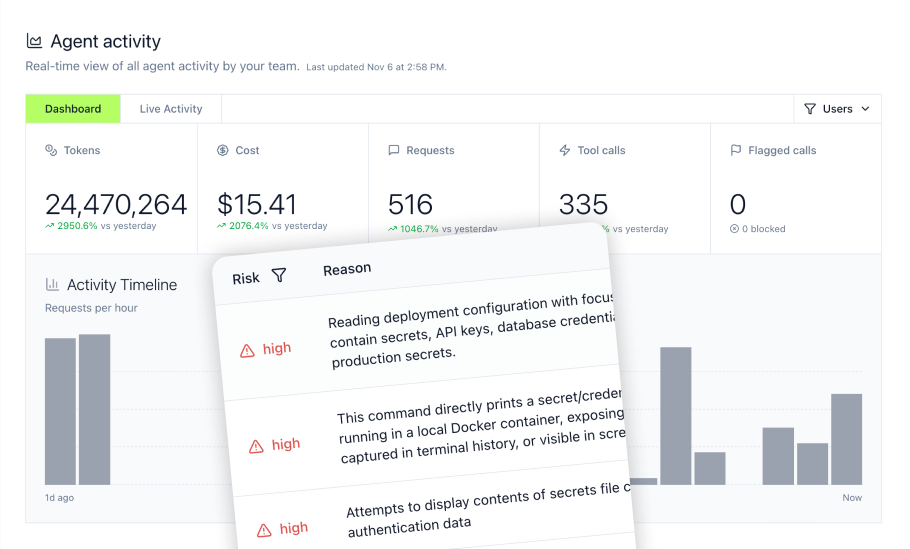
Deploying AI agents in pharmaceutical environments introduces security risks without proper governance. Coding agents like Cursor and Claude Code operate with extensive system access—reading files, executing commands, and accessing production databases through MCP tools. LLM Proxy provides the monitoring and control that pharmaceutical IT teams require.
Tracking AI agent access and blocking dangerous commands
The LLM Proxy monitors every interaction between coding agents and LLM providers, capturing MCP tool invocations (every database query, file read, API call executed through MCP servers), bash command history (complete audit trail of terminal commands), file access logs (which files the AI agent read or modified), and MCP inventory (visibility into installed MCP servers, permissions, and usage patterns).
Real-time security guardrails:
- Credential protection: Block AI access to
.envfiles, SSH keys, database passwords, and API tokens - Dangerous command blocking: Prevent execution of
rm -rf, file deletion, or system modification commands - Sensitive file restrictions: Protect access to configuration files, secrets management, and proprietary source code
- Production system safeguards: Restrict AI tool calls to development environments, blocking write access to production databases
Complete audit trails for regulatory inspections
Pharmaceutical organizations must demonstrate data integrity under 21 CFR Part 11, requiring complete records of system access and electronic record modifications. The combined MCP Gateway + LLM Proxy infrastructure provides user attribution (every AI interaction tied to individual developer identity through OAuth), timestamp accuracy (precise logging of when AI agents accessed data or modified files), data source traceability (complete record of which databases or file systems the AI queried), and output capture (storage of AI-generated code or analysis outputs for review).
Deployment timeline: 1-2 months for developer tool monitoring; 3-4 months for production MCP governance
Who benefits most: IT security teams, compliance officers, clinical data managers, and development teams requiring AI coding assistance
Implementation roadmap: Deploying MCP infrastructure
Successfully deploying MCP infrastructure in pharmaceutical environments requires phased implementation, addressing regulatory validation, stakeholder alignment, and change management.
Phase 1: Pilot with Non-Regulated data (months 1-3)
Objective: Demonstrate MCP value with minimal regulatory risk. Recommended pilot: literature monitoring or market intelligence using the Elasticsearch MCP Server.
Key activities: Select cross-functional pilot team, deploy MCP Gateway with OAuth and audit logging, configure Elasticsearch MCP Server, train 10-15 pilot users, establish success metrics.
Success criteria: Significant reduction in information retrieval time with high user satisfaction and zero compliance incidents.
Phase 2: Expand to regulated data (months 4-8)
Objective: Scale to GxP-regulated data sources with complete validation and compliance documentation.
Key activities: Conduct validation protocol development for computerized system compliance, perform data privacy impact assessment, configure granular tool access controls, implement human-in-the-loop review for all AI outputs affecting regulatory submissions, deploy LLM Proxy for coding agent monitoring, and document standard operating procedures.
Compliance deliverables: Validation summary report, risk assessment, user access management procedures, audit trail review procedures, and change control process.
Phase 3: Enterprise rollout (months 9-12)
Objective: Scale the MCP infrastructure across a pharmaceutical organization with multi-region deployment and continuous optimization.
Enterprise features: Multi-region MCP Gateway deployment with data residency controls, self-hosted option evaluation for highest-security use cases, cross-functional integration connecting MCP servers to a comprehensive data ecosystem, and advanced use cases (pharmacovigilance automation, commercial analytics, clinical trial operations).
Ongoing governance: Quarterly compliance audits, monthly security reviews, continuous monitoring of industry AI governance trends, and annual validation review ensuring computerized system compliance.
Organizational impact: Significant operational efficiency gains in information-intensive functions, faster decision cycles, and improved healthcare provider engagement quality.
Frequently asked questions
Q: How quickly can pharmaceutical brands deploy MCP infrastructure for AI agents?
Literature monitoring and market intelligence use cases can be deployed in several months for basic implementation, while complex applications requiring GxP validation (pharmacovigilance systems, clinical trial operations) need additional time, including regulatory compliance documentation.
Q: What compliance certifications does MCP gateway provide for pharmaceutical AI deployments?
MCP Gateway has completed a SOC 2 Type II audit when handling protected health information, providing complete audit trails supporting 21 CFR Part 11 regulatory compliance when implemented with appropriate validation and procedural controls. The platform offers data residency controls, ensuring clinical trial data and commercial information remain in approved geographic regions.
Q: Can MCP infrastructure integrate with legacy pharmaceutical systems?
Yes, though pharmaceutical companies operating decades-old systems may require custom MCP server development or middleware solutions to bridge technology gaps. MCP servers act as translation layers between legacy CTMS, EDC platforms, and modern AI clients, enabling standardized access without replacing existing validated systems.
Q: How does MCP gateway ensure audit trails meet pharmaceutical regulatory requirements?
MCP Gateway captures complete audit logs for every AI interaction, including user identity (through OAuth/SAML authentication), timestamp, data sources accessed, tool invocations executed, and AI outputs generated. This supports data integrity principles recognized in GxP guidance when implemented with appropriate validation and procedural controls. LLM Proxy adds monitoring of coding agent behavior, including bash commands, file access, and MCP tool usage, providing the comprehensive traceability that regulatory inspectors expect.
Q: What security controls does the MCP infrastructure provide for protecting pharmaceutical data?
MCP Gateway enforces OAuth and SAML authentication, requiring individual user credentials rather than shared service accounts, enabling precise attribution of all AI interactions. Granular tool access control allows configuring read-only database operations while blocking write, delete, or schema modification capabilities.